Auto logout in seconds.
Continue LogoutWe are in the midst of a significant transition in medicine from broad approaches to therapy to a more targeted, precise, and ultimately personalized approach. Core to this transition is our ability to identify actionable genetic information using lab-based biomarker tests. Biomarker testing helps providers make more informed treatment decisions based on a patient's genomic makeup or the genomic makeup of their cancer.
Additionally, biomarker testing can align with a health system's broader strategic goals including improving individual outcomes for patients, reducing total cost of care, and improving overall population health by allowing clinicians to give patients therapies most likely to lead to positive outcomes and minimize use of those unlikely to work for them.
Despite this promise, our research conversations make it clear that biomarker testing is not having the impact it could. We set out to estimate the current population receiving targeted cancer therapy for common cancers using publicly available data. Due to data limitations, we used the prevalence of positive biomarker tests as a proxy for the prevalence of each biomarker at the tumor-site level.
Our analyses found increased testing rates over time and increased targeted therapy. However, rates of testing for all biomarkers still need to improve, as does completion of testing prior to initiation of therapy. To learn more about our methodology, scroll to the end of this article.
Both patients and providers need to make informed decisions about care, but three things currently make this a challenge: inconsistent reimbursement and access, operational challenges across the care continuum, and low patient engagement.
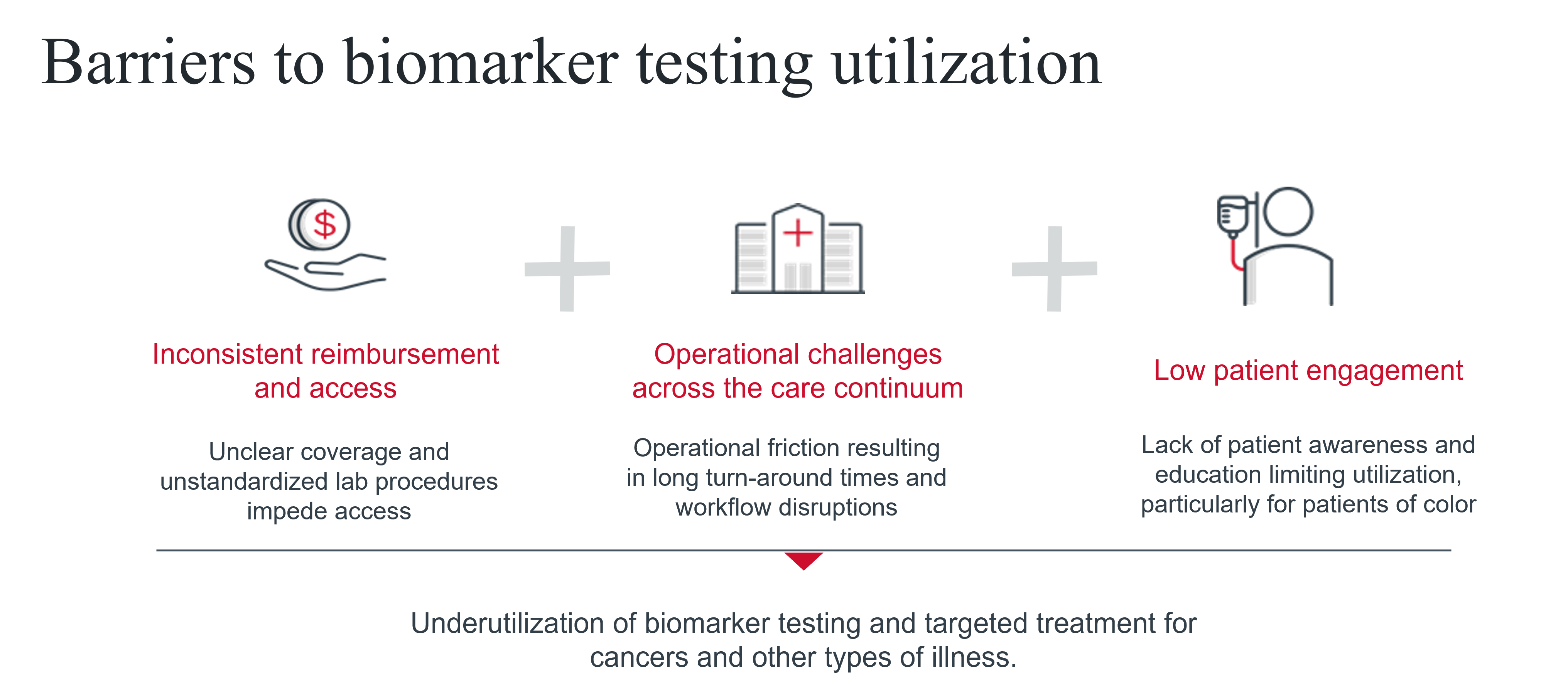
1. Inconsistent reimbursement and access
Reimbursement for biomarker testing is not consistent across commercial health plans. Biomarker testing coverage tends to be better under Medicare and large employer-sponsored plans. This could be because Medicare and large employer-sponsored plans are better funded and better able to manage risk pools across a large patient population.
The American Cancer Society also identified concerns about patient out-of-pocket costs and insurance coverage as significant barriers to provider utilization of biomarker testing. Inconsistencies in reimbursement cause challenges that lead to providers not ordering a biomarker test.
Additionally, reimbursement for biomarker testing is not consistent across therapeutic areas. Biomarker testing is routinely done to determine targeted treatment for patients with select types of cancer—including non-small cell lung cancer (NSCLC), breast cancer, and colorectal cancer.
However, other patients who could benefit from biomarker testing don't receive it. For example, in our analyses we found that just 14% of uterine cancer patients received biomarker testing compared to 55% of NSCLC patients. Universal biomarker testing is difficult for patients with other types of cancer and chronic diseases because assays are not standardized across labs.
Lack of standardized reimbursement across health plans and therapeutic areas creates broader access and equity challenges because patients with financial means can more easily and consistently navigate current financial hurdles to biomarker testing. Equitable progress and systematic access will require stakeholders to:
- Form multidisciplinary committees to make recommendations that align clinical value and payment structure for biomarker testing
- Call on policymakers to address financial barriers to biomarker testing such as coverage and out-of-pocket costs
- Work with patient advocacy groups to promote price transparency and help patients navigate the cost of biomarker testing
- Establish clear and consistent reimbursement guidelines across therapeutic areas to incentivize labs to standardize procedures
Improved reimbursement standards are necessary but will ultimately prove insufficient without clinician buy-in and organizational change. We need to adopt a treatment paradigm that embeds genetic testing upfront in care planning.
2. Operational challenges across the care continuum
Clinician buy-in is imperative as frontline clinicians are best positioned to implement biomarker testing and targeted treatment in patient care. Biomarker testing has become a common part of cancer management and its use is increasing across prevention, screening, and predictive procedures for other types of illness.
Clinicians must be equipped to implement this growing technology, but they often lack the expertise and time to do so. The novel and ever-changing nature of this field also makes it hard for clinicians to stay up to date on the latest evidence and implement it in their practice. To support widespread clinician adoption and improve biomarker testing rates, organizational change is needed.
Biomarker testing requires coordination across a range of clinical roles, including PCPs, oncologists, and lab technicians. Long turn-around times between testing and results can cause disconnects along the chain of communication meaning patients receive care plans that don't take advantage of genetic information and thus fail to receive indicated targeted therapies. Organizations need a clear communication plan that addresses challenges faced at each level to reduce turn-around times and decrease friction in the biomarker testing process.
Altering ingrained organizational practices and supporting clinician behavior change requires ongoing education and tools to support shared decision-making conversations with patients. An appropriate care team structure and processes must be in place to enable systematic change. Service line and clinical leaders can work together to raise clinician awareness and strengthen their biomarker testing programs by:
- Developing a cohesive message and vision around how biomarker testing advances clinical and strategic objectives
- Codifying processes and procedures to facilitate uptake of biomarker testing
- Identifying and supporting physicians and patients who are biomarker testing champions
- Establishing processes to regularly update clinicians on changing guidelines and evidence
- Designating teams responsible for patient follow up and invest in appropriate data infrastructure to track patient follow up and outcomes
- Creating lab partner strategy and structure workflows to give clinicians proper time to read and interpret biomarker testing results
- Designing evaluation and monitoring infrastructure that holds clinicians accountable to established guidelines
After education, a mature biomarker testing program should implement clinical decision support that can surface biomarker testing insights. Integrative, point-of-care solutions that bridge frontline clinicians with specialists and constantly update what genetic variations can be acted on, empowers clinicians to make real-time, informed treatment decisions alongside patients.
To realize the full potential of clinical decision support systems:
- Minimize alerts that interrupt and distract clinicians. Instead, provide clinicians with condition-specific guidance that makes it easier to recommend appropriate biomarker testing
- Incorporate multiple types of patient-specific data to improve data quality and boost value of CDS
- Manage the life cycle of knowledge to ensure regular review of biomarker guidelines and updates to keep CDS current
- Anticipate process changes and track unintended consequences to ensure the CDS system remains effective
- Hold senior executives accountable for working with IT to manage and govern CDS, permitting appropriate targeting, ownership, and selection of CDS mechanisms
Well executed coordination across the care continuum can help your biomarker program accomplish strategic goals by making the experience more efficient for clinicians and better for patients.
3. Low patient engagement
To make precision medicine a reality for patients, patients must be equipped to engage in shared decision-making conversations around biomarker testing. Lack of patient awareness and knowledge of biomarker testing limits the patients' ability to advocate for it to their providers and health plans, creating broader access and equity challenges.
Racial disparities in biomarker testing also persist—patients of color experience higher mortality rates for many cancers, but biomarker testing rates among patients of color are lower than white patients.
It is imperative to maintain a health equity lens and take action to increase awareness and access for marginalized communities. Organizations will need to adapt their messaging to engage these patients and help them understand how and when to advocate for biomarker testing as well as clarify misconceptions around it. Organizations can make their biomarker testing programs more accessible to marginalized communities by:
- Working with patient advocacy groups to develop a robust and accessible communications plan that communicates the value of biomarker testing for populations you want to reach
- Identifying and partnering with trust-brokers in the communities you want to reach to build patient and community trust around the collection, use, and protection of genomic information
- Prioritizing education and communication specific to building awareness and understanding among marginalized communities
- Identifying and alleviating barriers that marginalized communities face in participating in broader genomic research to create the diverse body of evidence needed to move biomarker testing forward
Ensuring that all groups can benefit equally from biomarker testing and other advancements in precision medicine requires stakeholders to work together to engage and educate patients of color.
Parting thoughts
We are developing novel, expensive, and potentially curative cell, and gene therapies for a broader range of conditions than the current focus on rare and orphan diseases. While these products are currently considered niche, they represent the future of therapeutic care. But patients won't reap the clinical, financial, and other benefits without stakeholders first increasing investment in a targeted approach to diagnostics.
The emphasis on treatment, rather than screening and early diagnosis, lead to downstream avoidable costs. Biomarker testing and targeted therapies can enable us to deliver precise, high-quality care, but we must empower providers to maximize this potential.
Our methodology
We estimated testing positivity based on the number and prevalence of each tumor site's biomarkers. We estimated biomarker testing rates based on published data for non-small cell lung cancer (NSCLC), which has one of the highest rates in oncology. We assigned equal or lower rates for other sites based on biomarker prevalence and number of FDA-approved companion diagnostics.
For this step, testing positivity is assumed to be accurate and therefore reflect prevalence (no false negatives or positives.) The number of patients receiving targeted therapy is calculated by applying a universal therapy rate to the number of eligible patients—those with a positive biomarker test. We sourced the testing rate from a 2017 study of 600,000 non-small cell lung cancer NSCLC patients. Below are the results of our study:
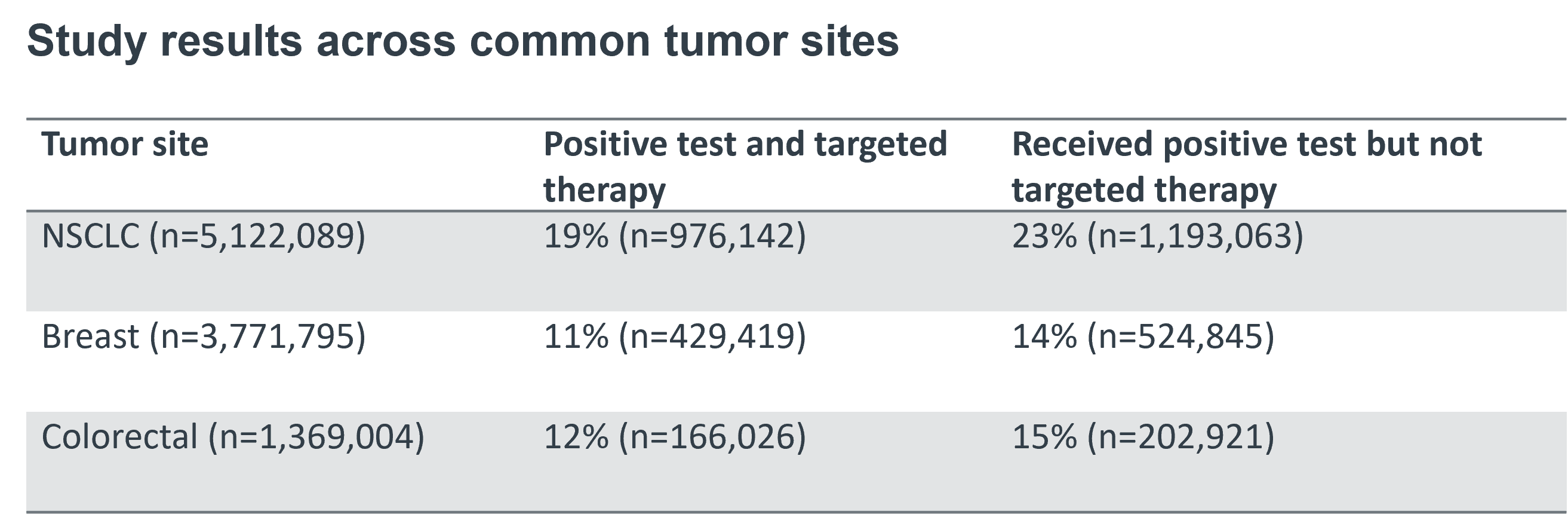
When adjusted for potential affected population if all tumor sites are tested at current rate of NSCLC (55%) and are placed on targeted therapy at an aggressive NSCLC rate (68%), the rate of patients who received a positive test but not targeted therapy decreased from 23% to 14%, 14% to 8%, and 15% to 9% for NSCLC, breast, and colorectal tumor sites respectively. Although our analyses found increased testing rates and targeted therapy over time, biomarker testing rates remain low.
![]() Download this watchlist to understand what healthcare leaders need to know about the evolving landscape.
Download this watchlist to understand what healthcare leaders need to know about the evolving landscape.
Don't miss out on the latest Advisory Board insights
Create your free account to access 1 resource, including the latest research and webinars.
Want access without creating an account?
You have 1 free members-only resource remaining this month.
1 free members-only resources remaining
1 free members-only resources remaining
You've reached your limit of free insights
Become a member to access all of Advisory Board's resources, events, and experts
Never miss out on the latest innovative health care content tailored to you.
Benefits include:
You've reached your limit of free insights
Become a member to access all of Advisory Board's resources, events, and experts
Never miss out on the latest innovative health care content tailored to you.
Benefits include:
This content is available through your Curated Research partnership with Advisory Board. Click on ‘view this resource’ to read the full piece.
Email ask@advisory.com to learn more.
Click on ‘Become a Member’ to learn about the benefits of a Full-Access partnership with Advisory Board
Never miss out on the latest innovative health care content tailored to you.
Benefits Include :
This is for members only. Learn more.
Become a member to access all of Advisory Board's resources, events, and experts
Never miss out on the latest innovative health care content tailored to you.



